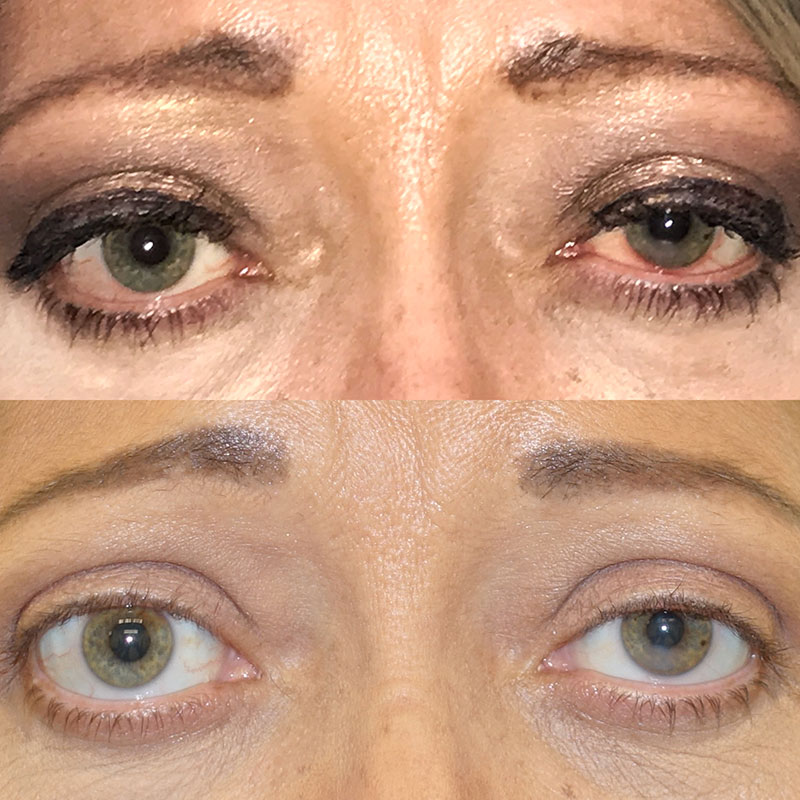
Tepezza (teprotumumab) is a monoclonal antibody medication which was FDA-approved in the US in the spring of 2020. Tepezza is the only medication which has been shown to permanently improve inflammation, proptosis, and double vision in patients with thyroid eye disease.

What is Tepezza?

Patient after 2 of 8 doses of Tepezza
Who is a candidate for Tepezza?
Currently, patients who have active thyroid eye disease and meet specific clinical criteria (clinical activity score >4) are a candidate for the drug. Tepezza may work better the earlier you start the drug, so do not delay getting a formal evaluation! However, Dr. Ramesh is part of ongoing studies for patients who have chronic thyroid eye disease (meaning they have had the disease for > 2 years). Patients may still benefit from being treated even if they have had disease for years. We encourage an evaluation with Dr. Ramesh to see if Tepezza is right for you.
What are common side effects?
Tepezza is not for patients with inflammatory bowel disease (Crohn’s or ulcerative colitis). The most common side effects included high blood sugar, muscle spasm, tinnitus (ringing ears), and gastrointestinal upset. 90% of patients who start Tepezza are able to finish the treatment without having to stop due to other side effects. Dr. Ramesh is also experienced in alternate dosing regimens to minimize the risk of side effects.
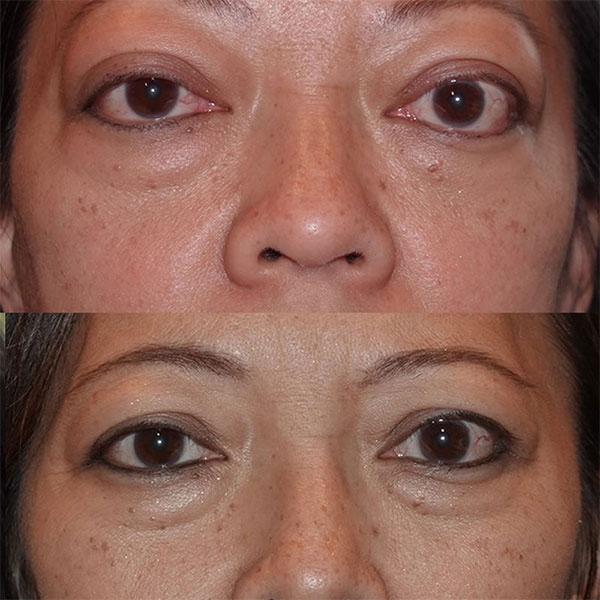
Patient after 4 of 8 doses of Tepezza
What is Dr. Ramesh's experience with Tepezza?
Dr. Ramesh was involved in the initial research for this medication prior to FDA approval, both in this time at UCLA as well as in his private practice at Wills Eye Hospital, and has extensive experience in treating patients with this drug both locally and in collaboration with other physicians for patients who travel from a distance.
Dr. Ramesh was also selected as one of the world experts in administration and treatment of thyroid eye disease with Tepezza by Horizon Therapeutics, the pharmaceutical company who manufactures Tepezza. In this role, Dr. Ramesh educates surgeons, endocrinologists, and primary eye care providers in the early diagnosis and state-of-the-art management of thyroid eye disease.
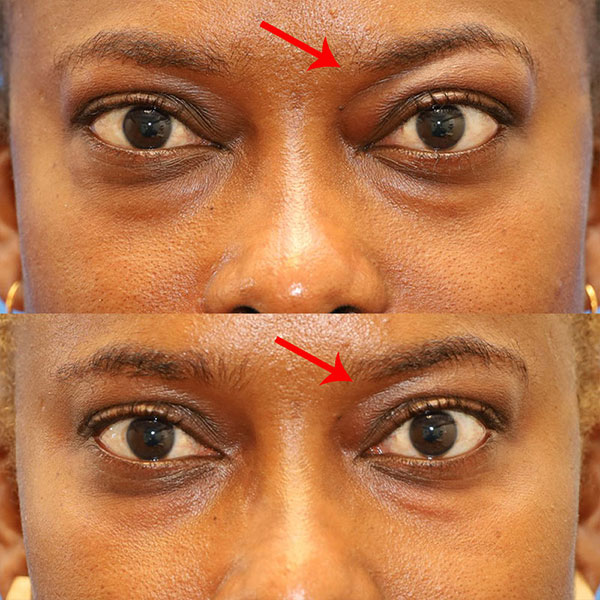
Patient with reduction in periorbital fat after just 1 of 8 doses of Tepezza
Are there ongoing research trials?
Yes, Dr. Ramesh is involved in ongoing research projects with Tepezza. Please contact our office to see if you are qualified for any research trials. We are currently engaged in research for all aspects of thyroid eye disease, including epidemiology, medical management, Tepezza, and surgical treatment.
Does insurance cover Tepezza?
Tepezza can be covered by health insurance for many patients. This depends on your medical history, severity of disease, and health insurance. Please contact our office to see if medical insurance would cover Tepezza for you.

Schedule an
Appointment
BOOK NOW
Contact Us

The Center for Eye and Facial Plastic Surgery
35 Clyde Road, #104
Somerset, NJ 08873
Monday – Friday: 8:30a – 4:30p
Livingston Office
22 Old Short Hills Rd Suite 202
Livingston, NJ 07039
Monday: 9:00a – 5:00p
Tuesday: 12:00p – 7:00p
Wednesday – Friday: 9:00a – 5:00p
P: (609) 608-0142
F: (855) 644-0469